Physics 207 - Ideal Gas Tutorial
Exercise 1
A cylinder contains an ideal gas that is at room temperature. The cylinder is sealed with a piston of mass M and cross-sectional area A that is free to move up or down without friction. No gas can enter or leave the cylinder. The piston is at rest. Atmospheric pressure is $P_0$.

On a separate sheet of paper, draw a free body diagram for the piston.
The label for each force should indicate:
- The type of force
- The object on which the force is exerted
- The object exerting the force
In the box below, indicate the direction of the net force on the piston. Explain your reasoning.
Is the force exerted on the piston by the gas inside the cylinder greater than, less than, or equal to the force exerted on the piston by the air outside the cylinder? Explain your reasoning.
Write an equation that relates all of the forces that are on your free body diagram.
Is the pressure of the gas inside the cylinder greater than, less than, or equal to atmospheric pressure? Explain your reasoning.
Determine the value of the pressure of the gas in the cylinder in terms of the given quantities.
A second cylinder contains a different sample of an ideal gas at room temperature as shown below. The 2 cylinders and their pistons are identical.

Is the pressure for the gas in the second cylinder greater than, less than, or equal to the pressure in the cylinder above? If you can not tell, state so explicitly. Explain.
Exercise 2
A cylinder of the type described in Exercise 1 contains a fixed amount of gas. Initially, it is in thermal equilibrium with an ice water bath. The pressure, volume, and temperature of the gas are $P_\textrm{initial}$, $V_\textrm{initial}$, and $T_\textrm{initial}$.
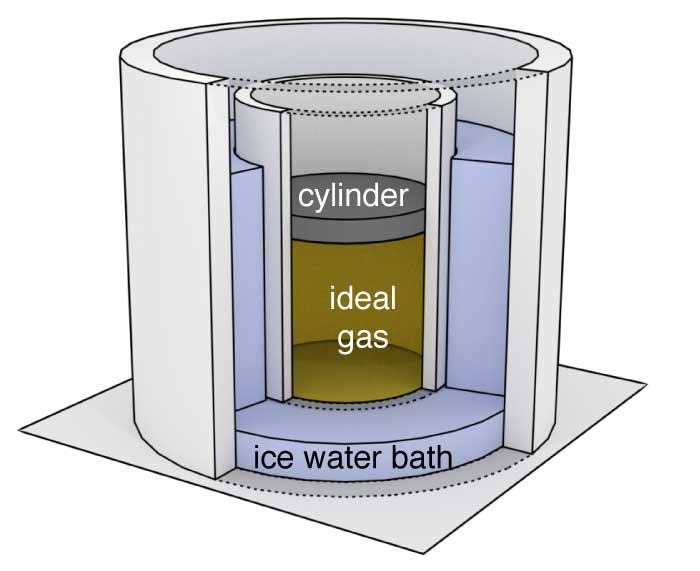
The cylinder is then removed from the ice water and placed into boiling water. After the system has come to thermal equilibrium with the boiling water, the pressure, volume, and temperature are $P_\textrm{final}$, $V_\textrm{final}$, and $T_\textrm{final}$.
Is $T_\textrm{final}$ greater than, less than, or equal to $T_\textrm{initial}$?
Is $P_\textrm{final}$ greater than, less than, or equal to $P_\textrm{initial}$?
Make sure that your answer is consistent with what you did in Exercise 1.
Is $V_\textrm{final}$ greater than, less than, or equal to $V_\textrm{initial}$?
Make sure that your answer is consistent with the ideal gas law ($PV = nRT$).
In the process above, which variables are held constant and which are allowed to change? Explain how you can tell.
Exercise 3
Ideal gas processes are often represented graphically. For instance, a $PV$ diagram is a graph of pressure versus volume for a given sample of gas. A single point on the graph represents simultaneously measured values of pressure and volume. These values define a state of the gas.
Sketch the process described in Exercise 2 on a PV diagram like the one below.
Label the initial and final states of the gas.
Exercise 4
The sample of ideal gas from exercise 2 is used for a new experiment. The pressure and volume of the gas are measured at several times. The values of $P$ and $V$ are recorded on the diagram below.

Rank the temperatures of the gas in the states A, B, C, and D from largest to smallest. If any 2 temperatures are the same, state so explicitly.
Make sure that your answer is consistent with the ideal gas law.
Is it possible for the gas to be in a state in which it has the same volume as in state B and the same temperature as in state A? If so, indicated the location of the state on the PV diagram. If not, explain why not.
Exercise 5
Two identical cylinders of the type described above contain hydrogen and oxygen. Both cylinders have been in the same room for a long time. Their pistons are at the same height.
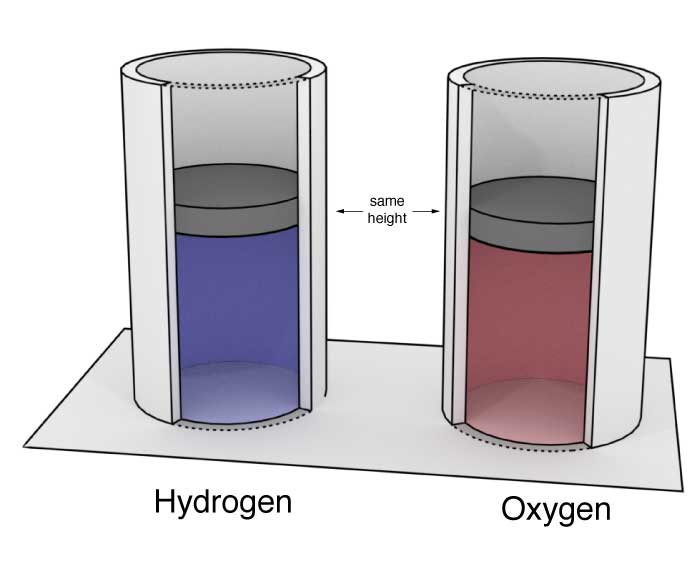
Compare the volumes of the gasses in the two cylinders. Explain.
Compare the temperatures in the two cylinders. Explain.
Compare the pressures in the two cylinders. Explain.
Compare the numbers of moles in the two cylinders. Explain.
Make sure that your answer is consistent with the ideal gas law.
A student looks up the molar masses and finds the values 2 g (for $H_2$) and 32 g (for $O_2$).
Give an interpretation of these 2 numbers. (Note that a formula is not considered an interpretation.)
Compare the masses of the gas samples in the 2 containers. Explain.
Exercise 6
A cylinder with a valve at the bottom is filled with an ideal gas. The valve is opened and some of the gas escapes slowly. The valve is then closed, after which the piston is observed to be at a lower position. Assume the system is in thermal equilibrium with the surroundings at all times.

Is the final pressure of the gas in the cylinder greater than, less than, or equal to the initial pressure? Explain.
In the process, which of the quantities P, V, n, and T are held constant and which are allowed to change?
Exercise 7
The piston in the following setup is fixed in place an unable to move up or down. The cylinder containing the ideal gas is then placed into boiling water.

Does the temperature of the gas increase, decrease, or remain the same? Explain.
Sketch this process on a $PV$ diagram.
Explain why for this particular situation it is not possible to determine the pressure of the gas you did in the other exercises.
Exercise 8
Two identical cylinders are closed by identical pistons that are free to move. Cylinder 1 contains oxygen gas. Cylinder 2 contains an amount of helium gas such that the two pistons are at the same height. The two gas samples are at equal temperatures. There are some small masses on top of each piston, but the number of masses on the piston closing cylinder 1 is greater.

Is the pressure of the oxygen in cylinder 1 greater than, less than, or equal to the pressure of the helium in cylinder 2? Explain your reasoning.
Is the number of moles of oxygen in cylinder 1 greater than, less than, or equal to the number of moles of helium in cylinder 2? Explain your reasoning.
The materials are based on activities from Tutorials in introductory physics, L.C. McDermott, P.S. Shaffer, and the Physics Education Group at the University of Washington (Prentice Hall, Upper Saddle River NJ, 1998).